My E.G. Services Berhad (MyEG) has a rapid testing kit for the COVID-19 disease (2019-nCoV) and is ready to deploy this in Malaysia and the Philippines. The testing kit, developed by a leading diagnostics company, is approved by the health authorities in China where it is presently widely used. MyEG is currently awaiting approval by local authorities for the use of the kits in Malaysia.
The MyEG rapid testing kit is a one-step test that allows for the qualitative detection of the 2019-Novel Coronavirus (SARS-CoV-2) IgM antibody in serum, plasma, fingertip blood or whole-body samples of pneumonitis patients or suspected cases.
MyEG intends to sell the testing kit for MYR99 (~USD23) or MYR990 (~USD230) for a pack of 10. This is affordable compared to other available options including the home screening service from DoctorOnCall that is priced at MYR700. Besides that, IHH Healthcare’s Gleneagles and Pantai hospitals also provide on-demand screening for MYR950, as does KPJ Healthcare Bhd’s Lablink and Qualitas Medical Group.
Each MyEG COVID-19 testing kit contains:
- 10 test/boxes
- Novel Coronavirus (2019-nCoV) IgM Antibody test card in a sealed pouch with desiccant
- Disposable pipet
- Sample diluent
- User manual
How it works
According to MyEG, the test uses an anti-human IgM antibody conjugated with colloidal gold and recombinant 2019-nCoV nucleocapsid protein (N protein) and spike protein (S protein) coated on different test lines respectively.
When a person is infected by 2019-nCoV, their immune system produces specific antibodies for the viral antigen. This usually happens within three to seven days after infection.
After the samples have been applied to the test strip, the gold-labelled anti-human IgM antibody will bind with IgM in the sample and form marked antigen-antibody complexes.
These complexes move to the test card detection zone by capillary action. Then, the marked antigen-antibody complexes will be captured on different test lines by recombinant 2019-nCoV N protein and S protein resulting in purplish red streaks on the test line. The colour intensity of each test line increases in proportion to the amount of 2019-nCoV IgM antibody in the sample.
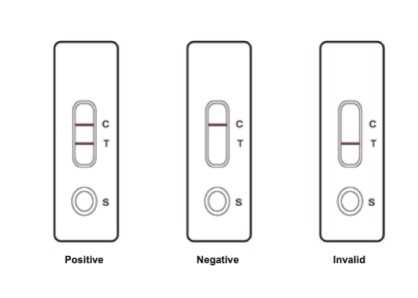
A positive result is indicated by two purplish red bands at the control area (C) and the test region (T). If only one purplish-red band appears at the control area (C), it indicates the result is negative. On the other hand, if no band appears on the control area (C), the test result is invalid. The test should then be repeated.
Check out the “how to” video from MyEG.
MyEG claims the kit is able to accurately detect the COVID-19 coronavirus within a span of 30 minutes without the need for nose or throat swabs, or for samples to be sent to external diagnostic laboratories.
The company is said to be collaborating with approved medical facilities to offer on-site testing with these kits.
Last month, MyEG unveiled an artificial intelligence-powered (AI) COVID-19 coronavirus risk profiling system. The smart health profiling and tracking system is capable of handling large volumes of travellers.